23+ How To Determine Rate Law
Rate kNOmO 3n We can determine the values of m n and k from the experimental data using the following three-part process. We can calculate the slope using any two points that lie on the line in the plot of ln N 2 O 5 versus t.

Rate Law Calculations When One Reactant Is Not Held Constant Youtube
Rate k NO2O2 Find the overall order of the reaction and the.
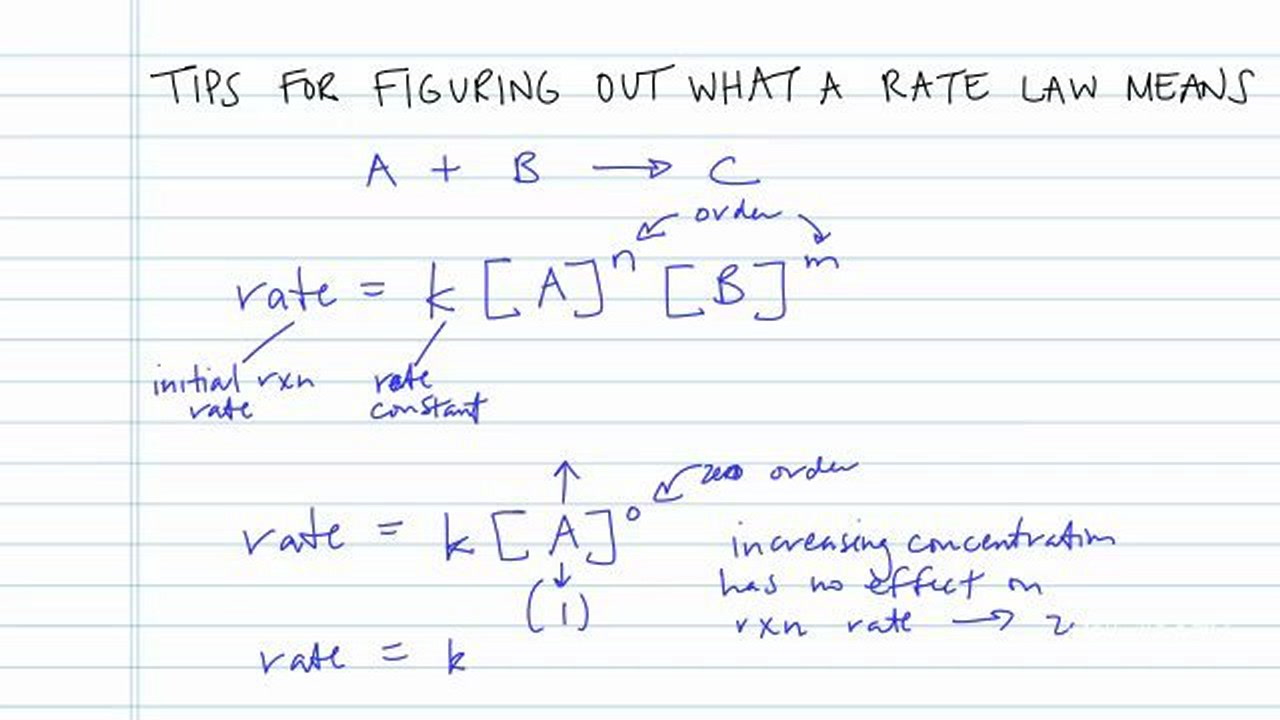
. The goal of a kinetics experiment is to measure the concentration of a species at a particular time during a reaction so that a rate law can be determined. For a reaction such as aA products the rate law generally has the form rate kAⁿ. Web Thus n must be 1 and the form of the rate law is.
Web rate k NO 2 H 2 The sum of the exponents is 2 1 3 making the reaction third-order overall. In the last three experiments NO varies while O 3 remains constant. Rate kNOmCl 2n kNO2Cl 2 Determine the numerical value of the rate constant k with appropriate units.
Once the rate law for a reaction is determined the specific. The comparison of experiments 2 and 3 is as follows. Determine the values of m n and k from the experimental data using the following three-part process.
Determine the value of m from the data in which NO varies and O3 is constant. Web The rate law is used to calculate the rate of reaction for any particular set of reactant concentrations. Web A rate law shows how the rate of a chemical reaction depends on reactant concentration.
Web The rate of a chemical reaction is determinedand alteredby many factors including the nature of reactivity of reactants surface area temperature concentration and catalysts. Web If Rate is given by k A x B y the overall order of the reaction n x y. Web The rate law will have the form.
Web The rate law for a chemical reaction can be determined using the method of initial rates which involves measuring the initial reaction rate at several different initial reactant. Web The rate law can be determined experimentally using the method of initial rates where the instantaneous reaction rate is measured immediately on mixing the reactants. It can figure out two things about a particular chemical reaction.
Determine the value of m from the. The order of a reaction is the sum of the powers of the concentrations of the reactants in. Web The rate law will have the form.
Web Solved Examples on the Rate Law Example 1 For the reaction given by 2NO O2 2NO2 The rate equation is. First we will find the reaction order of NO. Web B The rate law for the reaction is therefore rate k N 2 O 5 Calculating the rate constant is straightforward because we know that the slope of the plot of ln A versus t for a first-order reaction is k.
Determine the reaction order with respect to the first reactant. For each unique chemical reaction rate laws can be written.
Factors Of 23 Prime Factorization Methods Tree And Examples

Reaction Rates How To Determine Rate Law Chemtalk
Gdvgfmrgnjernm
Rate Constant Calculator Online Solver With Free Steps

How To Find The Rate Law For A Reaction With Initial Rate Data Worked Example By Hand Youtube

Determining Rate Equation Rate Law Constant Reaction Order From Experimental Data Video Lesson Transcript Study Com

Ppt Kinetics Chapter 14 Powerpoint Presentation Free Download Id 2069277

Measuring How Fast Reactions Occur Lecture 2 Stoichiometry Rate Laws Ppt Download

The Rate Law Concentration And Time Boundless Chemistry Course Hero

The Simulated A And Measured B 2d Avoided Crossing Spectrum Of The Download Scientific Diagram

The Rate Constant Of A First Order Reaction Whose Half Life Is 480 Seconds Is

Determining Rate Equation Rate Law Constant Reaction Order From Experimental Data Video Lesson Transcript Study Com

Rate Constant And Rate Laws Video Lesson Transcript Study Com
How To Determine The Order Of Reaction From Rate Law Quora

Measuring How Fast Reactions Occur Lecture 2 Stoichiometry Rate Laws Ppt Download

Rate Laws Determine The Rate Law From Experimental Data Explain The Effect Of Concentration On Reaction Rates Derive Rate Law Form A Reaction Ppt Download
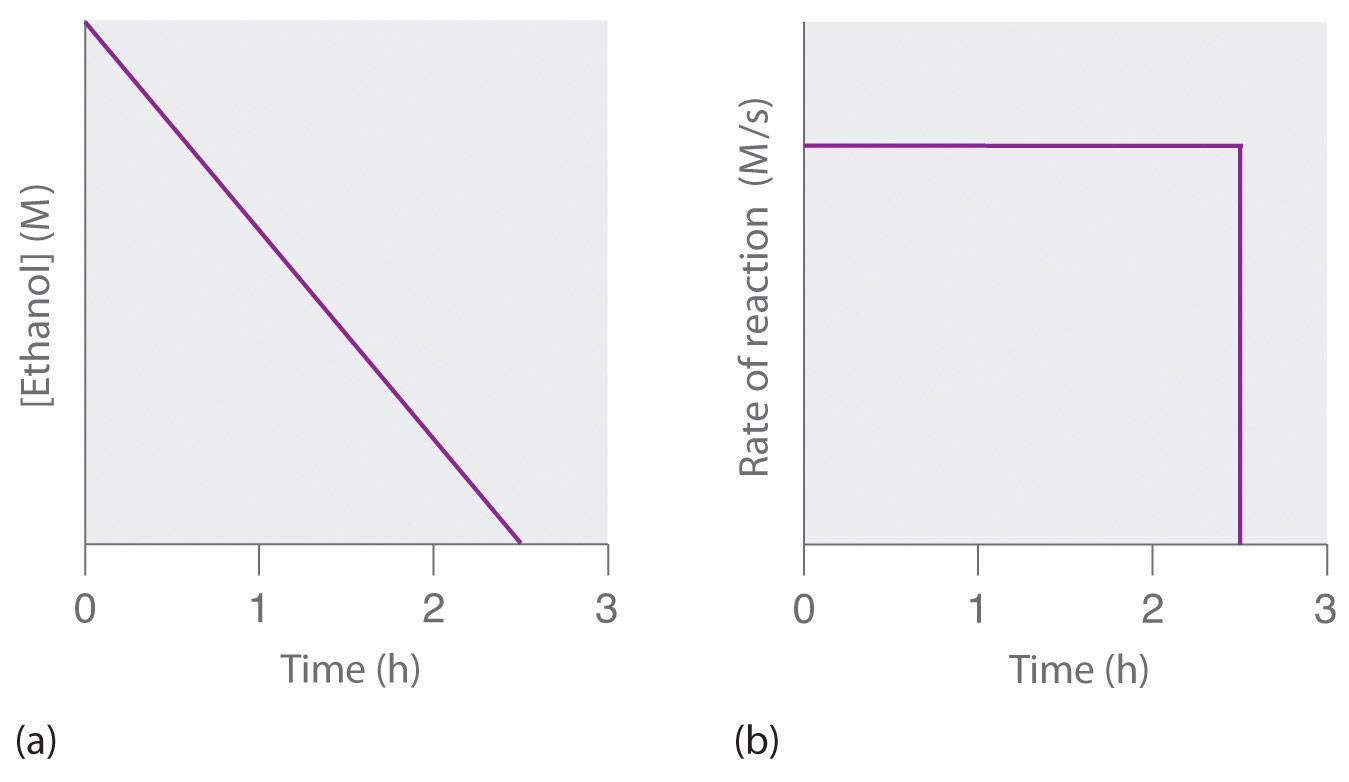
5 2 Methods Of Determining Reaction Order Chemistry Libretexts